Urea is the most widely used plant fertilizer, accounting for about 60 % of the global nitrogen fertilizer used in the World. It is prepared in huge quantities (ca. 210 Mt in 2022) from ammonia (produced via the energy expensive Haber-Bosh process from nitrogen and hydrogen) and from carbon dioxide. However, much of the urea nitrogen is lost through volatilization, denitrification and leaching. Dry urea deposited in the soil is degraded by the nickel-dependent enzyme urease. If the fertilizer is not incorporated with tillage, injected beneath the surface residue and rapidly irrigated, urease is able to quickly hydrolyze urea into hydrogen carbonate (HCO3–) and ammonium (NH4+) at a rate 1015 times faster than in the absence of urease. Although NH4+ serves as a nutrient to plants, the overall pH increase leads to the formation of gaseous ammonia, which is released in the atmosphere. Urease is not the only enzyme acting in the soil. The copper-dependent enzyme ammonia monooxygenase (AMO) present in ammonia-oxidizing microorganisms catalyzes the oxidation of NH3 into hydroxylamine (NH2OH),that is further oxidized to nitrite (NO2–) and nitrate (NO3–), which are precursors of gaseous NO and N2O and contribute to the greenhouse effect.
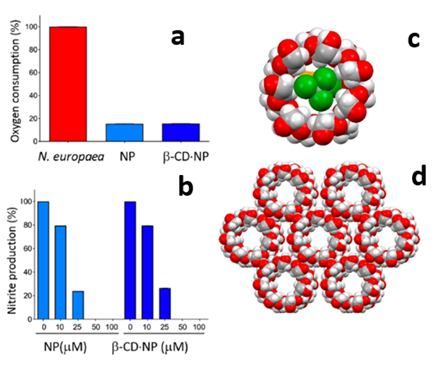
We are working on the crystal engineering – i.e. synthesis, characterization and evaluation – of a series of compounds to be used as inhibitors of the activity of the soil enzymes responsible for urea degradation. In collaboration with the groups of Stefano Luciano Ciurli (University of Bologna), Jonas Baltrusaitis (University of Leigh), and Franziska Emmerling (BAM, Berlin) we have reported on co-crystals and coordination compounds based on association of the fertilizer urea with enzyme inhibitors such as cathecol, zinc, and thiourea, and on the encapsulation (see figure) of urease inhibitors within cycledextrine in order to increase their solubility. The preparative method of choice is based on the mechanochemical mixing of the solid reactants.